February Dr. Rocks' Blog!
What makes the surface of ice allow fast skating by hockey players?
Answer: The surface of ice has a non-crystalline structure.
Notice the "shiny trail" left on ice by a skater. This shiny ribbon-like trail quickly disappears as the skater goes by. What is it? It is a trail of water. The skater was moving on a "wet ice thin film". There is disagreement among scientists as to why the surface of ice melts so easily under ice skates. Here are the major theories.
One explanation is that Ice melts under the high pressure exerted by the skater's ice skates. The high pressure was caused by the weight of the skater exerted over the small area of his ice skate blades.
Another explanation is that the notion of ice skates creates friction which heats and melts the ice under the ice skates.
Yet another explanation is that the surface of ice is non-crystalline in molecular structure. The surface molecules of ice pack down to form a thin film, which makes the ice slippery.
It is interesting that very cold ice has a rough surface which allows a person to walk on the ice in ordinary shoe's and not slip. Warmer ice, just several degrees below freeing, is too slippery to walk on in ordinary shoes.
More research is needed to understand the roles played by pressure, friction and the surface forces on ice. The method called "science" does not have all the answers at this time.
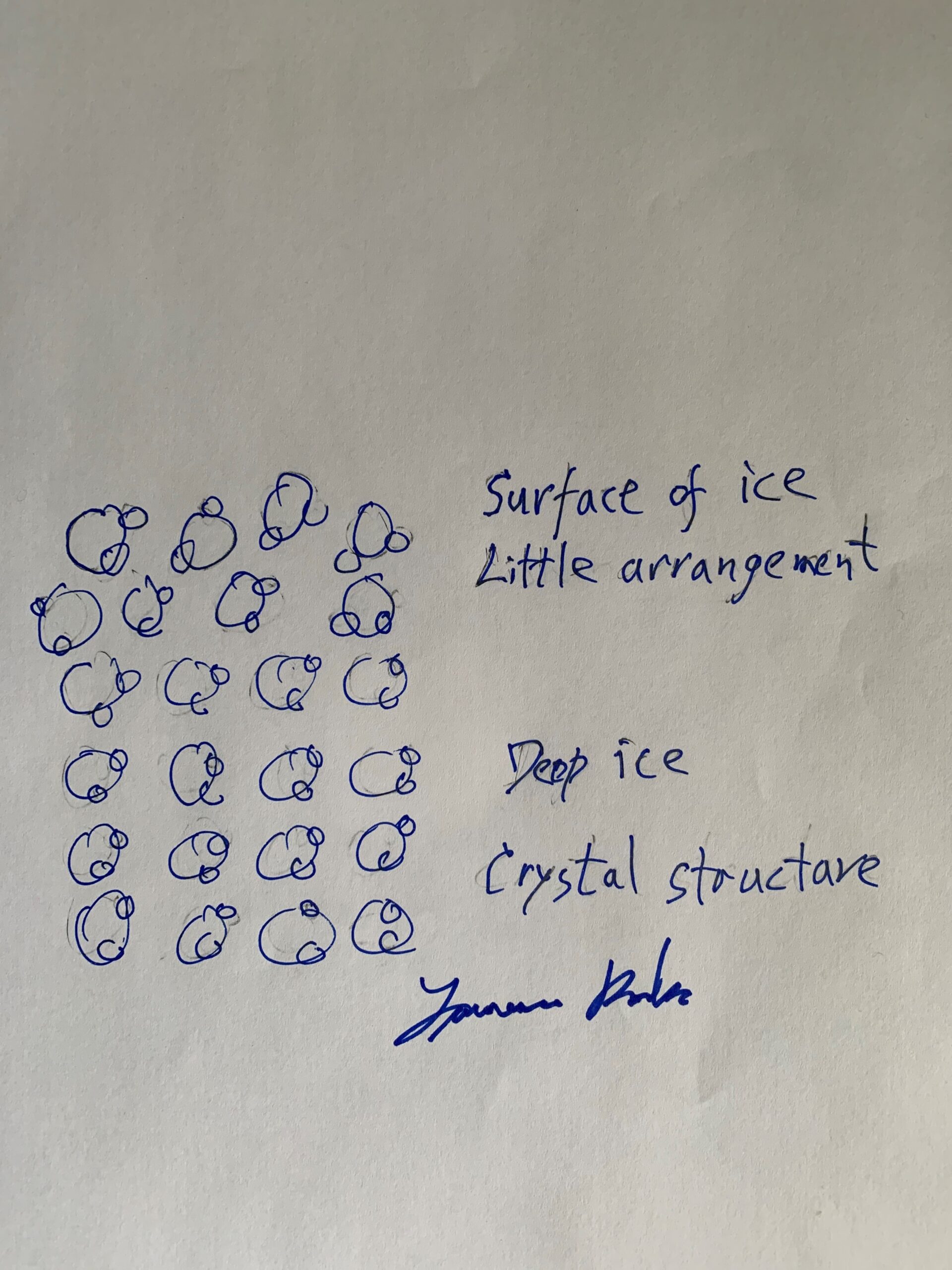
Picture: Water molecules at the surface of ice.

